Have you ever gotten an MRI scan in a hospital? It has probably been taken by a technique known as “three-dimensional Fourier-accelerated magnetic resonance tomography”. In essence, your body went into a giant test tube and was analyzed by magnetic resonance spectroscopy of its nuclear spins. While this technique - nuclear magnetic resonance spectroscopy - usually informs about chemical species in the test tube, it can be turned into an imaging technique by applying a magnetic field gradient. If spectroscopy is performed in a spatially varying magnetic field – a field that is, say, larger in your head than in your feet – spins in different parts of the body will emit signals at different frequencies – a larger one in the head, a lower one in the feet. The spectrum of the nuclei becomes a one-dimensional image. Applying gradients along different directions of space will render it three-dimensional. Crucially, the gradient trick by itself would not be sufficient to make clinical imaging viable. Spectroscopy also has to be performed at the highest possible speed. The data volume of a three-dimensional scan is enormous, and even with the fastest spectroscopy sequences available today, you probably had to hold still for several minutes in the scanner. That it has been minutes rather than hours is in fact rather surprising, and only possible thanks to massively parallel acquisition techniques, which can receive the signal from every pixel of the image simultaneously in a single scan. These protocols are based on the clever use of a Fourier transform, and require rapid and exact switching of the magnetic field gradients, which generates the debilitating noise you probably heard during your scan.
In the past decade, novel magnetic field detectors made from diamond have made it possible to perform magnetic resonance spectroscopy on much smaller samples than before. Even single biomolecules can now be analyzed by nuclear and electron spin resonance spectroscopy.
This discovery has sparked dreams of also applying magnetic resonance imaging to nanoscale samples to deliver a magnetic resonance microscope with atomic resolution, essentially a miniature version of a clinical MRI scanner, only a million times smaller.
Yet, turning nanoscale magnetic resonance from a mere spectroscopy to an imaging technique has remained an elusive goal so far.
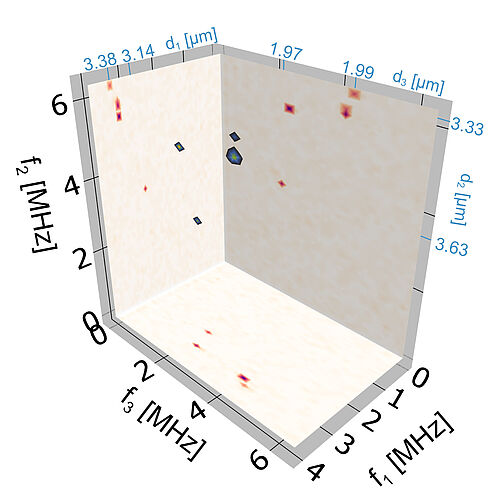
Our work provides one essential component for the journey towards this goal. We demonstrate a switchable electromagnet, made from lithographically fabricated gold nanowires, which can provide magnetic field gradients along all three directions of space, and can switch these gradients sufficiently quickly and precisely that Fourier-acceleration becomes possible and large three-dimensional volumes can be imaged. We demonstrate this by imaging single nitrogen atoms (precisely: “nitrogen-vacancy centers”) in a diamond sample in three dimensions with a resolution of better than 10 nanometers. By optimising fabrication to shrink the size of the nanowire layout by another order of magnitude, this can likely be pushed to the sub-nanometer scale, comparable to the size of single atoms.
While our work, to our best knowledge, sets a world record as the highest-resolution three-dimensional Fourier magnetic resonance image ever obtained, it only is one step on a long journey. The sample imaged in our proof-of-concept experiments is the sensor diamond itself. Applying the technique to real-life samples of electron and nuclear spins, living outside the diamond, will be the next challenge. If successful, the most powerful optical and electron microscopy techniques in the world would be rivalled by magnetic resonance imaging – a technique once considered notoriously insensitive and thus intrinsically limited to large samples like humans.
Original paper: npj Quantum Information 10, 16 (2024)
Contact:
University of Rostock
AG Quantum Technology
Prof. Friedemann Reinhard
☎ +49 381 4986840
@ friedemann.reinharduni-rostockde
? https://www.qt.physik.uni-rostock.de/en/